Temporomandibular Joint Disorders
Functional abnormalities of the temporomandibular joints are probably the most common findings one observes when examining a patient for masticatory dysfunction. The reason for this is due to the high prevalence of signs, and not necessarily symptoms. Many of the signs such as joint sounds or deviated opening are not painful, and therefore the patient may not seek treatment. These TM joint disorders generally fall into two broad categories: Internal derangements and inflammatory joint disorders. These conditions will be described separately.
Internal Derangements
Internal derangements represent a group of functional disorders that arise from abnormalities in the anatomy and/or positional relationships of the TM joint structures. A review of TMJ anatomy demonstrates that the disc is attached to the poles of the condyle by the medial and lateral collateral ligaments. Many internal derangement disorders arise from alterations of the integrity or lengths of these ligamentous attachments. Once these ligaments become elongated the disc is allowed more freedom to move within the joint. The disc will often begin to assume an anteromedial position in relationship with the condyle (Fig. 2.4). When the disc is in this more forward and medial position, function of the joint can be somewhat altered. As the mouth opens and the condyle moves forward, a short distance of translatory movement can occur between the condyle and the disc until the condyle once again assumes its normal position on the thinnest area of the disc (intermediate zone). Once it has translated over the posterior surface of the disc to the intermediate zone, inter-articular pressure due to joint loading maintains this relationship and the disc is again carried forward with the condyle through the remaining portion of the translatory movement. Upon closing, the disc reassumes its abnormal position on the condyle in the closed joint position. Once in the closed joint position, the disc is again free to move according to the demands of its functional attachments. In this condition, the disc will assume the most anteromedial position allowed by the discal attachments and its own morphology.
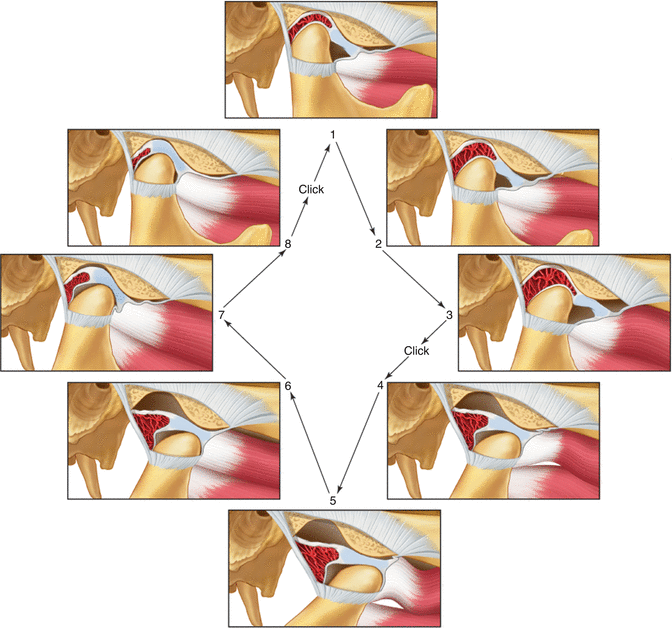
Fig. 2.4
Functional displacement of the disc with reduction. Note that while the mouth is closed (stage 1) the disc is displaced anterior to the condyle. During opening the condyle passes over the posterior border of the disc onto the intermediate area of the disc, thus reducing the displaced disc (stage 4). At this stage a click is felt. During the rest of the opening movement the condyle and disc function normally. During closing the disc is re-displaced (stage 8–1) and a second click is felt (reciprocal click) (From Okeson [2], p. 145)
As the disc is more chronically repositioned forward and medially by action of the superior lateral pterygoid muscle, the discal ligaments are further elongated. With continuous thinning of the posterior border of the disc and further elongation of the disc ligaments, the disc can move through the discal space and be trapped anterior to the condyle. (Fig. 2.4). When this occurs, the condyle can now function or load the retrodiscal tissues which may be associated with pain.
The important feature of this functional relationship is that the condyle translates across the disc to some degree when movement begins. This type of movement does not occur in the normal joint. During such movement the increased interarticular pressure may prevent the articular surfaces from sliding across each other smoothly. The disc can stick or be bunched slightly, causing an abrupt movement of the condyle over it into the normal condyle-disc relationship. A clicking sound often accompanies this abrupt movement. Once the joint has clicked, the normal relationship of the disc and condyle is reestablished and this relationship is maintained during the rest of the opening movement. A second click can occur as the disc is re-displaced during the later stages of closing the mouth. This is called “reciprocal clicking” [50]. As the disc displacement progresses, the condyle actually begins to function behind the disc with loading occurring on the retrodiscal tissues (Fig. 2.4).
As the disc is more chronically repositioned forward and medially, the discal ligaments are further elongated. With continuous thinning of the posterior border of the disc and further elongation of the disc ligaments, the disc can move further forward and be trapped anterior to the condyle. This often is accompanied by folding of the disc into a ball-like shape. When this occurs it will initially lead to a decrease in how far the condyle can move forward. Therefore the patient will have the sensation that he or she cannot open the mouth completely. This has been called a “closed lock” (Fig. 2.5) [50] since the patient feels he or she is locked near the closed mouth position. Patients may report pain when the mandible is moved to the point of limitation, but pain does not always accompany this condition [51–54].
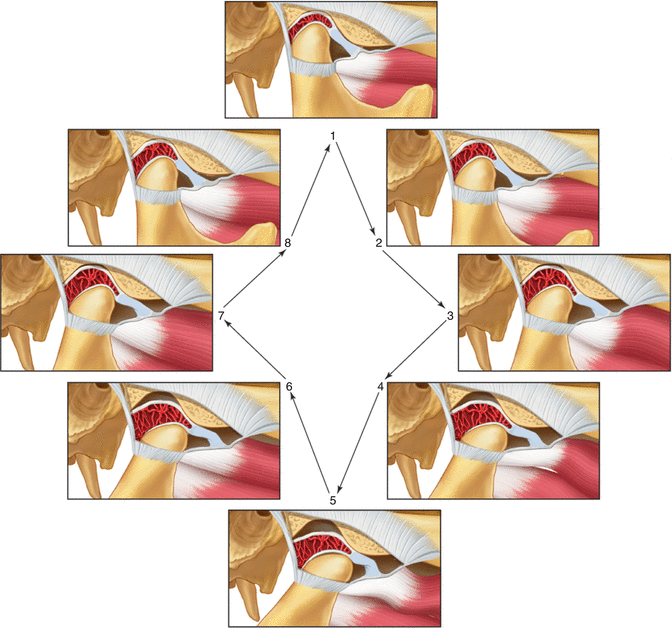
Fig. 2.5
Functional displacement of the disc without reduction Note that during opening the condyle never assumes a normal relationship on the disc but instead causes the disc to move forward ahead of it. This condition limits the distance it can translate forward (closed lock). Clicking often resolves when this occurs (From Okeson [2], p. 146)
If a closed lock continues, the condyle will be constantly positioned on the retrodiscal tissues. These tissues are not anatomically structured to accept forces, but they often remodel to form a functional pseudodisc. However, as force is applied, some likelihood arises that these tissues may break down in some patients [55–57]. With this breakdown comes tissue inflammation and pain (retrodiscitis).
It is important to appreciate that pain is not always a factor with these conditions. Pain is not generated by the disc, since it is aneural. The structures that are able to produce pain are the connective tissues such as the ligaments and the very highly innervated retrodiscal tissues. If these structures are loaded quickly due to a sudden disc shifting, pain is likely. However, if the changes occur slowly over time, these tissues are often able to adapt and pain may not be associated.
Etiology of Internal Derangements
Any condition or event that leads to elongation of the discal ligaments or thinning of the disc can cause these derangements of the condyle-disc complex disorders. Certainly one of the most common factors is trauma. Two general types of trauma need to be considered: macrotrauma and microtrauma. Macrotrauma relates to a sudden blow to the face that can result in a quick elongation of ligaments. This is well documented in the literature [58–71].
Microtrauma represents lower levels of force but repeated over longer periods of time. It can result from joint loading associated with muscle hyperactivity such as bruxism or clenching [72, 73]. This may be especially true if the bruxing activity is intermittent and the tissues have not had an opportunity to adapt. It is likely that if the bruxing is long standing, the articular tissues have adapted to the loading forces and changes will not be seen. In fact, in most patients gradual loading of the articular surfaces leads to an adaptive, more tolerant articular tissue [74–76].
Microtrauma may be the result of mandibular loading in the presence of orthopedic instability, as stated in an earlier section. When orthopedic instability exists, repeated loading, such as clenching the teeth, can lead to slight movements of the condyle resulting in microtrauma to the ligaments. The results can be elongation of these ligaments and eventually disc movements. Remember that the amount and intensity of the loading greatly influence whether the orthopedic instability will lead to a disc derangement disorder. Bruxing patients with orthopedic instability, therefore, are more likely to develop problems than non-bruxers with the same occlusion.
An important question that arises in dentistry is “What occlusal conditions are commonly associated with internal derangements?” The most orthopedically stable position of the condyle is in the superior anterior position resting against the posterior slope of the articular eminence. This is referred to as the musculoskeletally stable position [2, p. 291–316] and it is determined by the loading forces of the elevator muscles. It has been demonstrated that when an occlusal condition causes a condyle to be positioned posterior to the musculoskeletally stable position the posterior border of the disc can be thinned [77]. A common occlusal condition that has been suggested by some orthodontists to produce this problem is the skeletal Class II deep-bite malocclusion; advocates of this concept also believe that this situation may be further aggravated when a Division 2 anterior relationship also exists [78–82]. However, most studies show no relationship between Class II malocclusion and these disorders [13, 83–89]. Other studies show no association between the horizontal and vertical relationship of the anterior teeth and disc derangement disorders [90–94]. While occlusal conditions are not the main etiologic factors for internal derangements, the important feature of an occlusal condition that leads to disc derangement disorders is the lack of joint stability when the teeth are tightly occluded. Therefore, it is likely that some Class II malocclusions provide joint stability (a stable malocclusion) while others do not; but the same can be said for every type of static malocclusion category.
It is obvious that no simple relationship exists between orthopedic instability and intracapsular disorders. It is very important, however, that when orthopedic instability exists, it be identified as a potential etiologic factor. It should be noted that orthodontic therapy can be a viable treatment for orthopedic instability and may need to be considered when this instability has been determined to be a contributing factor to a TMD.
2.3.2.2 Osteoarthritis
When internal derangements of the TMJ occur, the structures affected by these changes often respond. The most common tissues affected are the retrodiscal tissues and the articular surfaces of the condyle and articular eminence. These changes can result in adaptation or destruction, depending upon many factors. Some of these factors are the acuteness of the changes as well as the intensity and duration of the loading. There are also important biologic and genetic factors that may regulate the patient’s ability to repair tissues.
If the articular surfaces of the condyle become affected, the subarticular bone receives additional loading and changes can occur. Similar changes also can occur in the absence of internal derangements. These changes represent a group of disorders that are considered joint arthritides. The most common type of TMJ arthritis is osteoarthritis (sometimes called degenerative joint disease). Osteoarthritis represents a destructive process by which the bony articular surfaces of the condyle and fossa become altered. It is generally considered to be the body’s response to increased loading of a joint [95]. As loading forces continue and the articular surface becomes softened (chondromalacia), the subarticular bone begins to resorb. Progressive degeneration eventually results in loss of the subchondral cortical layer, bone erosion, and subsequent radiographic evidence of osteoarthritis [96]. It is important to note that radiographic changes are only seen in later stages of osteoarthritis and may not reflect the clinical symptoms accurately.
Osteoarthritis is often painful, and jaw movement accentuates the symptoms. Crepitation (multiple grating joint sounds) is a common finding with this disorder. Osteoarthritis can occur any time the joint is overloaded, but is most commonly associated with disc displacements [97, 98] or perforation [99]. Once the disc is displaced and the retrodiscal tissues break down, the condyle begins to articulate directly with the fossa accelerating the destructive process. In time, the dense fibrous articular surfaces are destroyed and bony changes occur. Radiographically, the surfaces seem to be eroded and flattened. Any movement of these surfaces creates pain, so jaw function usually becomes very restricted. Although osteoarthritis is in the category of inflammatory disorders, it is not a true inflammatory condition. With appropriate treatment and reduction of joint loading, the arthritic condition can become adaptive. The adaptive stage has been referred to as osteoarthrosis [95, 100].
Other types of arthritides can certainly affect the temporomandibular joint. The most common after osteoarthritis is rheumatoid arthritis. This is thought to be an autoimmune disorder and therefore has its origin in systemic factors. The juvenile form of this problem can produce significant joint changes as well as serious occlusal problems. Other common causes of TMJ arthritis are traumatic arthritis, infectious arthritis, psoriatic arthritis, and hyperuricemia (gout). These conditions are not reviewed in this chapter. Other texts can be reviewed for a more thorough review of TMJ arthritides [2, p. 317–61].
2.4 Summary of the Continuum of TMJ Intracapsular Conditions
Disorders of the temporomandibular joints may follow a path of progressive events, i.e., a continuum, from the initial signs of dysfunction to osteoarthritis. They are summarized in Fig. 2.6.
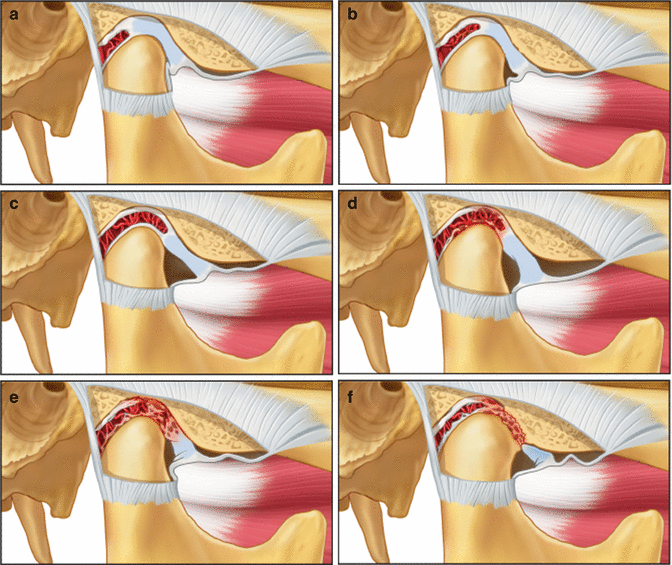
Fig. 2.6
Various states of internal derangement of the TMJ. (a) normal joint, (b) partial displacement of the disc, (c) complete displacement of the disc, (d) impingement of retrodiscal tissues, (e) retrodiscitis and tissue breakdown, (f) osteoarthritis (From Okeson [2], p. 156)
Although this continuum is logical, the question must be asked whether these stages are always progressive for every patient. It is a question of great significance, because if all patients continue to progress in this manner, then there would be a professional obligation to resolve any joint symptoms as soon as they first appear. The sequence of breakdown as summarized in Fig. 2.6 is logical and has clinical support [101–103]. However, clinical longitudinal studies have clearly shown that some intracapsular TMD patients will present in one stage but may not necessarily progress to the next. At any given stage of disc derangement the patient may reach a level of adaptability and no further progression or breakdown will occur [104, 105]. This can be supported by histories of asymptomatic single and reciprocal clicks over many years [106]. Perhaps the key to determining who needs treatment lies in the obvious progression from one stage to the next. Also the presence of pain is important, since it implies continuous breakdown; in any case, the pain should be treated for its own sake. Therefore, it is this author’s opinion that treatment for such patients needs to be instituted when pain is associated with the condition. The treatment should be directed toward controlling pain and changing loading, thereby allowing a better opportunity for the tissues to repair and eventually adapt. Treatment of these conditions is beyond the objectives of this chapter and therefore other sources should be pursued.
Conclusion
Temporomandibular disorders are common conditions that may be encountered by orthodontists: during pre-treatment screening, during their treatment procedures, or during orthodontic retention. Every orthodontist needs to have a basic understanding of these musculoskeletal disorders so that he or she can respond to their patients’ needs. The purpose of this chapter was to present the pathophysiology, etiology, and clinical characteristics of the most common temporomandibular disorders. The goal was not to provide detailed therapeutic considerations for TMDs. However, it should be mentioned that most TMDs can be successfully managed by very conservative, reversible treatments. This should always be the initial approach before any irreversible treatments are considered.
Although some patients with TMDs may respond to orthodontic therapy, most will not because their occlusal conditions are not the cause of their symptomatology. Also, the positive responses seen may simply be due to placebo effects, spontaneous improvements, or the passage of time. Therefore, the orthodontist needs to understand which TMD patients will benefit before any orthodontic therapy is begun, and that treatment should not be initiated until acute symptoms of pain and dysfunction have been addressed. A review of the five etiologic factors presented in this chapter reveals that orthodontic therapy potentially affects only one of them, i.e., the occlusal condition. The manner by which orthodontic therapy affects this factor is by providing orthopedic stability, because proper orthodontic therapy can provide a stable occlusal position in the most stable joint position. Accomplishing this provides orthopedic stability in the masticatory structures, which minimizes risk factors associated with TMDs. Since orthodontic treatment will always disrupt the existing occlusal and TMJ relationships, establishing this orthopedic stability at the end of treatment should be the goal for every patient who receives orthodontic therapy.
When a patient presents to the orthodontist with a TMD, the etiology of the TMD needs to be determined before any treatment is begun. Assuming that the patient’s malocclusion is the major etiologic factor causing the TMD is a very naïve assumption. Nothing is more discouraging to the patient (and doctor) than to provide excellent orthodontic treatment for 2 years and then hear the patient report that the pain is still present. Although the orthodontic therapy may have been successful, the orthodontist has failed to successfully treat the patient. Therefore, before beginning orthodontic therapy on any symptomatic patient, the clinician needs to confirm that orthopedic instability is an etiology for that patient, because this is the only scenario in which orthodontics can be considered as an appropriate treatment for TMD.
Take Home Messages
- TMD signs and symptoms are common in the general population, but only a small percentage of those require treatment.
- Orthodontists need to be aware how their treatments can affect masticatory function.
- There are five recognized etiologic factors associated with TMD.
- Muscle pain is the most common painful TMD encountered in the orthodontic practice.
- Internal derangements are associated with TMJ conditions and joint sounds are the most common sign.
- Most TMD symptoms can be managed by conservative approaches.
- Treatment goals for all orthodontists should include developing or maintaining orthopedic stability in the masticatory system.
References
1.
de Leeuw R, Glasser G. Orofacial pain: guidelines for classification, assessment, and management. 5th ed. Chicago: Quintessence Publ. Co.; 2013.
2.
Okeson JP. Management of temporomandibular disorders and occlusion. 7th ed. St. Louis: Elsevier/Mosby Publishers; 2013.
3.
De Kanter RJAM, Kayser AF, Battistuzzi PGFCM, Truin GJ, Van T, Hol MA. Demand and need for treatment of craniomandibular dysfunction in the Dutch adult population. J Dent Res. 1992;71:1607–12.PubMed
4.
Schiffman EL, Fricton JR, Haley DP, Shapiro BL. The prevalence and treatment needs of subjects with temporomandibular disorders. J Am Dent Assoc. 1990;120(3):295–303.PubMed
5.
Pullinger AG, Seligman DA. The degree to which attrition characterizes differentiated patient groups of temporomandibular disorders. J Orofac Pain. 1993;7(2):196–208.PubMed
6.
Carlson CR, Okeson JP, Falace DA, Nitz AJ, Curran SL, Anderson DT. Comparison of psychologic and physiologic functioning between patients with masticatory muscle pain and matched controls. J Orofac Pain. 1993;7:15–22.PubMed
7.
Grassi C, Passatore M. Action of the sympathetic system on skeletal muscle. Ital J Neurol Sci. 1988;9(1):23–8.PubMed
8.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 13–40.
9.
Kobs G, Bernhardt O, Kocher T, Meyer G. Oral parafunctions and positive clinical examination findings. Stomatologija/issued by public institution “Odontologijos studija” [et al]. 2005;7(3):81–3.
10.
Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain Off J Am Pain Soc. 2011;12(11 Suppl):T92–101.
11.
Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol Disord Drug Targets. 2012;11(3):222–35.PubMedCentralPubMed
12.
Slade GD, Diatchenko L, Ohrbach R, Maixner W. Orthodontic treatment, genetic factors and risk of temporomandibular disorder. Semin Orthod. 2008;14(2):146–56.PubMedCentralPubMed
13.
McNamara Jr JA, Seligman DA, Okeson JP. Occlusion, orthodontic treatment, and temporomandibular disorders: a review. J Orofac Pain. 1995;9(1):73–90.PubMed
14.
McCreary CP, Clark GT, Merril RL, et al. Psychological distress and diagnostic subgroups of temporomandibular disorder patients. Pain. 1991;44:29–34.PubMed
15.
Keele KD. A physician looks at pain. In: Weisenberg M, editor. Pain; clinical and experimental perspectives. St Louis: The CV Mosby Co; 1975. p. 45–52.
16.
Svensson P, Graven-Nielsen T. Craniofacial muscle pain: review of mechanisms and clinical manifestations. J Orofac Pain. 2001;15(2):117–45.PubMed
17.
Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7(6):419–25.PubMed
18.
Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil. 2008;89(1):157–9.PubMed
19.
Mense S. Algesic agents exciting muscle nociceptors. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2009;196(1):89–100.PubMed
20.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 41–54.
21.
Lund JP, Widmer CG. Evaluation of the use of surface electromyography in the diagnosis, documentation, and treatment of dental patients. J Craniomandib Disord. 1989;3(3):125–37.PubMed
22.
Lund JP, Widmer CG, Feine JS. Validity of diagnostic and monitoring tests used for temporomandibular disorders [see comments]. J Dent Res. 1995;74(4):1133–43.PubMed
23.
Paesani DA, Tallents RH, Murphy WC, Hatala MP, Proskin HM. Evaluation of the reproducibility of rest activity of the anterior temporal and masseter muscles in asymptomatic and symptomatic temporomandibular subjects. J Orofac Pain. 1994;8:402–6.PubMed
24.
Curran SL, Carlson CR, Okeson JP. Emotional and physiologic responses to laboratory challenges: patients with temporomandibular disorders versus matched control subjects. J Orofac Pain. 1996;10(2):141–50.PubMed
25.
Mense S. Considerations concerning the neurobiological basis of muscle pain. Can J Physiol Pharmacol. 1991;69(5):610–6.PubMed
26.
Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54(3):241–89.PubMed
27.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 287–326.
28.
Watanabe M, Tabata T, Huh JI, Inai T, Tsuboi A, Sasaki K, et al. Possible involvement of histamine in muscular fatigue in temporomandibular disorders: animal and human studies. J Dent Res. 1999;78(3):769–75.PubMed
29.
Christensen LV, Mohamed SE, Harrison JD. Delayed onset of masseter muscle pain in experimental tooth clenching. J Prosthet Dent. 1982;48(5):579–84.PubMed
30.
Abraham WM. Factors in delayed muscle soreness. Med Sci Sports. 1977;9:11–20.PubMed
31.
Tegeder L, Zimmermann J, Meller ST, Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res Off J Europ Histamine Res Soc [et al]. 2002;51(8):393–402.
32.
Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev. 1991;19:99–125.PubMed
33.
Byrnes WC, Clarkson PM. Delayed onset muscle soreness and training. Clin Sports Med. 1986;5(3):605–14.PubMed
34.
Bobbert MF, Hollander AP, Huijing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18(1):75–81.PubMed
35.
Bakke M, Michler L. Temporalis and masseter muscle activity in patients with anterior open bite and craniomandibular disorders. Scand J Dent Res. 1991;99(3):219–28.PubMed
36.
Tzakis MG, Dahlstrom L, Haraldson T. Evaluation of masticatory funciton before and after treatment in patients with craniomandibular disorders. J Craniomandib Disord Facial Oral Pain. 1992;6:267–72.
37.
Sinn DP, de Assis EA, Throckmorton GS. Mandibular excursions and maximum bite forces in patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 1996;54(6):671–9.PubMed
38.
High AS, MacGregor AJ, Tomlinson GE. A gnathodynanometer as an objective means of pain assessment following wisdom tooth removal. Br J Maxillofac Surg. 1988;26:284.
39.
Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985;60(6):615–23.PubMed
40.
Simons DG, Travell J. Myofascial trigger points, a possible explanation [letter]. Pain. 1981;10(1):106–9.PubMed
41.
Mense S, Meyer H. Bradykinin-induced sensitization of high-threshold muscle receptors with slowly conducting afferent fibers. Pain. 1981;(Suppl 1):S204.
42.
Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75(1):1–17.PubMed
43.
McMillan AS, Blasberg B. Pain-pressure threshold in painful jaw muscles following trigger point injection. J Orofac Pain. 1994;8(4):384–90.PubMed
44.
Travell J. Introductory comments. In: Ragan C, editor. Connective tissues transactions of the fifth conference. New York: Josiah Macy, Jr; 1954. p. 12–22.
45.
Simons DG, Travell JG, Simons LS. Travell & Simons’ myofascial pain and dysfunction: a trigger point manual. 2nd ed. Baltimore: Williams & Wilkins; 1999. p. 67–78.
46.
Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18(13):1803–7.PubMed
47.
Fernandez-de-Las-Penas C, Galan-Del-Rio F, Alonso-Blanco C, Jimenez-Garcia R, Arendt-Nielsen L, Svensson P. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disorders. J Pain Off J Am Pain Soc. 2010;11(12):1295–304.
48.
Giunta JL, Kronman JH. Orofacial involvement secondary to trapezius muscle trauma. Oral Surg Oral Med Oral Pathol. 1985;60(4):368–9.PubMed
49.
Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. 2000;131(9):1307–15.PubMed
50.
Farrar WB, McCarty Jr WL. The TMJ dilemma. J Ala Dent Assoc. 1979;63(1):19–26.PubMed
51.
Roberts CA, Tallents RH, Espeland MA, Handelman SL, Katzberg RW. Mandibular range of motion versus arthrographic diagnosis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1985;60(3):244–51.PubMed
52.
Tallents RH, Hatala M, Katzberg RW, Westesson PL. Temporomandibular joint sounds in asymptomatic volunteers. J Prosthet Dent. 1993;69:298–304.PubMed
53.
Dibbets JMH, van der Weele LT. The prevalence of joint noises as related to age and gender. J Craniomandib Disord Facial Oral Pain. 1992;6:157–60.
54.
Katzberg RW, Westesson PL, Tallents RH, Drake CM. Anatomic disorders of the temporomandibular joint disc in asymptomatic subjects. J Oral Maxillofac Surg. 1996;54:147–53.PubMed
55.
Isberg A, Isacsson G, Johansson AS, Larson O. Hyperplastic soft-tissue formation in the temporomandibular joint associated with internal derangement. A radiographic and histologic study. Oral Surg Oral Med Oral Pathol. 1986;61(1):32–8.PubMed
56.
Holumlund AB, Gynther GW, Reinholt FP. Disk derangement and inflammatory changes in the posterior disk attachment of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1992;73:9.
57.
Taskaya-Yylmaz N, Ogutcen-Toller M. Clinical correlation of MRI findings of internal derangements of the temporomandibular joints. Br J Oral Maxillofac Surg. 2002;40(4):317–21.PubMed
58.
Harkins SJ, Marteney JL. Extrinsic trauma: a significant precipitating factor in temporomandibular dysfunction. J Prosthet Dent. 1985;54(2):271–2.PubMed
59.
Moloney F, Howard JA. Internal derangements of the temporomandibular joint. III. Anterior repositioning splint therapy. Aust Dent J. 1986;31(1):30–9.PubMed
60.
Weinberg S, Lapointe H. Cervical extension-flexion injury (whiplash) and internal derangement of the temporomandibular joint. J Oral Maxillofac Surg. 1987;45(8):653–6.PubMed
61.
Pullinger AG, Seligman DA. Trauma history in diagnostic groups of temporomandibular disorders. Oral Surg Oral Med Oral Pathol. 1991;71(5):529–34.PubMed
62.
Westling L, Carlsson GE, Helkimo M. Background factors in craniomandibular disorders with special reference to general joint hypermobility, parafunction, and trauma. J Craniomandib Disord. 1990;4(2):89–98.PubMed
63.
Pullinger AG, Seligman DA. Association of TMJ subgroups with general trauma and MVA. J Dent Res. 1988;67:403.
64.
Pullinger AG, Monteriro AA. History factors associated with symptoms of temporomandibular disorders. J Oral Rehabil. 1988;15:117.PubMed
65.
Skolnick J, Iranpour B, Westesson PL, Adair S. Prepubertal trauma and mandibular asymmetry in orthognathic surgery and orthodontic paients. Am J Orthod Dentofacial Orthop. 1994;105:73–7.PubMed
66.
Braun BL, DiGiovanna A, Schiffman E, et al. A cross-sectional study of temporomandibular joint dysfunction in post-cervical trauma patients. J Craniomandib Disord Facial Oral Pain. 1992;6:24–31.
67.
Burgess J. Symptom characteristics in TMD patients reporting blunt trauma and/or whiplash injury. J Craniomandib Disord. 1991;5(4):251–7.PubMed
68.
De Boever JA, Keersmaekers K. Trauma in patients with temporomandibular disorders: frequency and treatment outcome. J Oral Rehabil. 1996;23(2):91–6.PubMed
69.
Yun PY, Kim YK. The role of facial trauma as a possible etiologic factor in temporomandibular joint disorder. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2005;63(11):1576–83.
70.
Arakeri G, Kusanale A, Zaki GA, Brennan PA. Pathogenesis of post-traumatic ankylosis of the temporomandibular joint: a critical review. Br J Oral Maxillofac Surg. 2012;50(1):8–12.PubMed
71.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 327–69.
72.
Israel HA, Diamond B, Saed Nejad F, Ratcliffe A. The relationship between parafunctional masticatory activity and arthroscopically diagnosed temporomandibular joint pathology. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 1999;57(9):1034–9.
73.
Nitzan DW. Intraarticular pressure in the functioning human temporomandibular joint and its alteration by uniform elevation of the occlusal plane. J Oral Maxillofac Surg. 1994;52(7):671–9.PubMed
74.
Milam SB, Schmitz JP. Molecular biology of temporomandibular joint disorders: proposed mechanisms of disease. J Oral Maxillofac Surg. 1995;12:1448–54.
75.
Monje F, Delgado E, Navarro MJ, Miralles C, Alonso Jr dH. Changes in temporomandibular joint after mandibular subcondylar osteotomy: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:1221–34.PubMed
76.
Shaw RM, Molyneux GS. The effects of induced dental malocclusion on the fibrocartilage disc of the adult rabbit temporomandibular joint. Arch Oral Biol. 1993;38:415–22.PubMed
77.
Isberg A, Isacsson G. Tissue reactions associated with internal derangement of the temporomandibular joint. A radiographic, cryomorphologic, and histologic study. Acta Odontol Scand. 1986;44(3):160–4.PubMed
78.
Wright Jr WJ. Temporomandibular disorders: occurrence of specific diagnoses and response to conservative management. Clin Observ Craniol. 1986;4(2):150–5.
79.
Seligman DA, Pullinger AG. Association of occlusal variables among refined TM patient diagnostic groups. J Craniomandib Disord. 1989;3(4):227–36.PubMed
80.
Solberg WK, Bibb CA, Nordstrom BB, Hansson TL. Malocclusion associated with temporomandibular joint changes in young adults at autopsy. Am J Orthod. 1986;89(4):326–30.PubMed
81.
Tsolka P, Walter JD, Wilson RF, Preiskel HW. Occlusal variables, bruxism and temporomandibulae disorder: a clinical and kinesiographic assessment. J Oral Rehabil. 1995;22:849–956.PubMed
82.
Celic R, Jerolimov V. Association of horizontal and vertical overlap with prevalence of temporomandibular disorders. J Oral Rehabil. 2002;29(6):588–93.PubMed
83.
Williamson EH, Simmons MD. Mandibular asymmetry and its relation to pain dysfunction. Am J Orthod. 1979;76(6):612–7.PubMed
84.
DeBoever JA, Adriaens PA. Occlusal relationship in patients with pain-dysfunction symptoms in the temporomandibular joint. J Oral Rehabil. 1983;10:1–7.
85.
Brandt D. Temporomandibular disorders and their association with morphologic malocclusion in children. In: Carlson DS, McNamara JA, Ribbens KA, editors. Developmental aspects of temporomandibular joint disorders. Ann Arbor: University of Michigan Press; 1985. p. 279.
86.
Nilner M. Functional disturbances and diseases of the stomatognathic system. A cross-sectional study. J Pedod. 1986;10(3):211–38.PubMed
87.
Stringert HG, Worms FW. Variations in skeletal and dental patterns in patients with structural and functional alterations of the temporomandibular joint: a preliminary report. Am J Orthod. 1986;89(4):285–97.PubMed
88.
Gunn SM, Woolfolk MW, Faja BW. Malocclusion and TMJ symptoms in migrant children. J Craniomandib Disord. 1988;2(4):196–200.PubMed
89.
Dworkin SF, Huggins KH, LeResche L, Von KM, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120(3):273–81.PubMed
90.
Ronquillo HI, et al. Comparison of internal deranagements with condyle-fossa relationship, horizontal and vertical overlap, and angle class. J Craniomandib Disord Facial Oral Pain. 1988;2:137.
91.
Pullinger AG, Seligman DA, Solberg WK. Temporomandibular disorders. Part II: occlusal factors associated with temporomandibular joint tenderness and dysfunction. J Prosthet Dent. 1988;59(3):363–7.PubMed
92.
Pullinger AG, Seligman DA. Overbite and overjet characteristics of refined diagnositic groups of temporomandibular disorders patients. Am J Orthod Dentofacial Orthop. 1991;100:401.PubMed
93.
Hirsch C, John MT, Drangsholt MT, Mancl LA. Relationship between overbite/overjet and clicking or crepitus of the temporomandibular joint. J Orofac Pain. 2005;19(3):218–25.PubMed
94.
John MT, Hirsch C, Drangsholt MT, Mancl LA, Setz JM. Overbite and overjet are not related to self-report of temporomandibular disorder symptoms. J Dent Res. 2002;81(3):164–9.PubMed
95.
Stegenga B, de Bont L, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg. 1989;47(3):249–56.PubMed
96.
Stegenga B, de Bont LG, Boering G, van Willigen JD. Tissue responses to degenerative changes in the temporomandibular joint: a review. J Oral Maxillofac Surg. 1991;49(10):1079–88.PubMed
97.
DeBont LGM, Boering G, Liem RSB, Eulderink F, Westesson PL, et al. Osteoarthritis and internal derangement of the temporomandibular joint: a light microscopic study. J Oral Maxillofac Surg. 1986;44:634–43.
98.
Mills DK, Daniel JC, Herzog S, Scapino RP. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillofac Surg. 1994;52(12):1279–92.PubMed
99.
Helmy E, Bays R, Sharawy M. Osteoarthrosis of the temporomandibular joint following experimental disc perforation in Macaca fascicularis. J Oral Maxillofac Surg. 1988;46(11):979–90.PubMed
100.
Boering G. Temporomandibular joint arthrosis: a clinical and radiographic investigation [thesis]. Groningen: University of Groningen; 1966.
101.
Farrar WB, McCarty WL. A clinical outline of temporomandibular joint diagnosis and treatment. 7th ed. Montgomery: Normandie Publications; 1983. p. 191.
102.
McCarty WL, Farrar WB. Surgery for internal derangements of the temporomandibular joint. J Prosthet Dent. 1979;42(2):191–6.PubMed
103.
Wilkes CH. Arthrography of the temporomandibular joint in patients with the TMJ pain-dysfunction syndrome. Minn Med. 1978;61(11):645–52.PubMed
104.
Akerman S, Kopp S, Rohlin M. Histological changes in temporomandibular joints from elderly individuals. An autopsy study. Acta Odontol Scand. 1986;44(4):231–9.PubMed
105.
Kircos LT, Ortendahl DA, Mark AS, Arakawa M. Magnetic resonance imaging of the TMJ disc in asymptomatic volunteers. J Oral Maxillofac [spoiler title=’Title’]Text[/spoiler] Surg. 1987;45(10):852–4.PubMed
106.
Magnusson T, Egermark I, Carlsson GE. A longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of age. J Orofac Pain. 2000;14(4):310–9.PubMed
Temporomandibular Joint Disorders
Functional abnormalities of the temporomandibular joints are probably the most common findings one observes when examining a patient for masticatory dysfunction. The reason for this is due to the high prevalence of signs, and not necessarily symptoms. Many of the signs such as joint sounds or deviated opening are not painful, and therefore the patient may not seek treatment. These TM joint disorders generally fall into two broad categories: Internal derangements and inflammatory joint disorders. These conditions will be described separately.
Internal Derangements
Internal derangements represent a group of functional disorders that arise from abnormalities in the anatomy and/or positional relationships of the TM joint structures. A review of TMJ anatomy demonstrates that the disc is attached to the poles of the condyle by the medial and lateral collateral ligaments. Many internal derangement disorders arise from alterations of the integrity or lengths of these ligamentous attachments. Once these ligaments become elongated the disc is allowed more freedom to move within the joint. The disc will often begin to assume an anteromedial position in relationship with the condyle (Fig. 2.4). When the disc is in this more forward and medial position, function of the joint can be somewhat altered. As the mouth opens and the condyle moves forward, a short distance of translatory movement can occur between the condyle and the disc until the condyle once again assumes its normal position on the thinnest area of the disc (intermediate zone). Once it has translated over the posterior surface of the disc to the intermediate zone, inter-articular pressure due to joint loading maintains this relationship and the disc is again carried forward with the condyle through the remaining portion of the translatory movement. Upon closing, the disc reassumes its abnormal position on the condyle in the closed joint position. Once in the closed joint position, the disc is again free to move according to the demands of its functional attachments. In this condition, the disc will assume the most anteromedial position allowed by the discal attachments and its own morphology.

Fig. 2.4
Functional displacement of the disc with reduction. Note that while the mouth is closed (stage 1) the disc is displaced anterior to the condyle. During opening the condyle passes over the posterior border of the disc onto the intermediate area of the disc, thus reducing the displaced disc (stage 4). At this stage a click is felt. During the rest of the opening movement the condyle and disc function normally. During closing the disc is re-displaced (stage 8–1) and a second click is felt (reciprocal click) (From Okeson [2], p. 145)
As the disc is more chronically repositioned forward and medially by action of the superior lateral pterygoid muscle, the discal ligaments are further elongated. With continuous thinning of the posterior border of the disc and further elongation of the disc ligaments, the disc can move through the discal space and be trapped anterior to the condyle. (Fig. 2.4). When this occurs, the condyle can now function or load the retrodiscal tissues which may be associated with pain.
The important feature of this functional relationship is that the condyle translates across the disc to some degree when movement begins. This type of movement does not occur in the normal joint. During such movement the increased interarticular pressure may prevent the articular surfaces from sliding across each other smoothly. The disc can stick or be bunched slightly, causing an abrupt movement of the condyle over it into the normal condyle-disc relationship. A clicking sound often accompanies this abrupt movement. Once the joint has clicked, the normal relationship of the disc and condyle is reestablished and this relationship is maintained during the rest of the opening movement. A second click can occur as the disc is re-displaced during the later stages of closing the mouth. This is called “reciprocal clicking” [50]. As the disc displacement progresses, the condyle actually begins to function behind the disc with loading occurring on the retrodiscal tissues (Fig. 2.4).
As the disc is more chronically repositioned forward and medially, the discal ligaments are further elongated. With continuous thinning of the posterior border of the disc and further elongation of the disc ligaments, the disc can move further forward and be trapped anterior to the condyle. This often is accompanied by folding of the disc into a ball-like shape. When this occurs it will initially lead to a decrease in how far the condyle can move forward. Therefore the patient will have the sensation that he or she cannot open the mouth completely. This has been called a “closed lock” (Fig. 2.5) [50] since the patient feels he or she is locked near the closed mouth position. Patients may report pain when the mandible is moved to the point of limitation, but pain does not always accompany this condition [51–54].

Fig. 2.5
Functional displacement of the disc without reduction Note that during opening the condyle never assumes a normal relationship on the disc but instead causes the disc to move forward ahead of it. This condition limits the distance it can translate forward (closed lock). Clicking often resolves when this occurs (From Okeson [2], p. 146)
If a closed lock continues, the condyle will be constantly positioned on the retrodiscal tissues. These tissues are not anatomically structured to accept forces, but they often remodel to form a functional pseudodisc. However, as force is applied, some likelihood arises that these tissues may break down in some patients [55–57]. With this breakdown comes tissue inflammation and pain (retrodiscitis).
It is important to appreciate that pain is not always a factor with these conditions. Pain is not generated by the disc, since it is aneural. The structures that are able to produce pain are the connective tissues such as the ligaments and the very highly innervated retrodiscal tissues. If these structures are loaded quickly due to a sudden disc shifting, pain is likely. However, if the changes occur slowly over time, these tissues are often able to adapt and pain may not be associated.
Etiology of Internal Derangements
Any condition or event that leads to elongation of the discal ligaments or thinning of the disc can cause these derangements of the condyle-disc complex disorders. Certainly one of the most common factors is trauma. Two general types of trauma need to be considered: macrotrauma and microtrauma. Macrotrauma relates to a sudden blow to the face that can result in a quick elongation of ligaments. This is well documented in the literature [58–71].
Microtrauma represents lower levels of force but repeated over longer periods of time. It can result from joint loading associated with muscle hyperactivity such as bruxism or clenching [72, 73]. This may be especially true if the bruxing activity is intermittent and the tissues have not had an opportunity to adapt. It is likely that if the bruxing is long standing, the articular tissues have adapted to the loading forces and changes will not be seen. In fact, in most patients gradual loading of the articular surfaces leads to an adaptive, more tolerant articular tissue [74–76].
Microtrauma may be the result of mandibular loading in the presence of orthopedic instability, as stated in an earlier section. When orthopedic instability exists, repeated loading, such as clenching the teeth, can lead to slight movements of the condyle resulting in microtrauma to the ligaments. The results can be elongation of these ligaments and eventually disc movements. Remember that the amount and intensity of the loading greatly influence whether the orthopedic instability will lead to a disc derangement disorder. Bruxing patients with orthopedic instability, therefore, are more likely to develop problems than non-bruxers with the same occlusion.
An important question that arises in dentistry is “What occlusal conditions are commonly associated with internal derangements?” The most orthopedically stable position of the condyle is in the superior anterior position resting against the posterior slope of the articular eminence. This is referred to as the musculoskeletally stable position [2, p. 291–316] and it is determined by the loading forces of the elevator muscles. It has been demonstrated that when an occlusal condition causes a condyle to be positioned posterior to the musculoskeletally stable position the posterior border of the disc can be thinned [77]. A common occlusal condition that has been suggested by some orthodontists to produce this problem is the skeletal Class II deep-bite malocclusion; advocates of this concept also believe that this situation may be further aggravated when a Division 2 anterior relationship also exists [78–82]. However, most studies show no relationship between Class II malocclusion and these disorders [13, 83–89]. Other studies show no association between the horizontal and vertical relationship of the anterior teeth and disc derangement disorders [90–94]. While occlusal conditions are not the main etiologic factors for internal derangements, the important feature of an occlusal condition that leads to disc derangement disorders is the lack of joint stability when the teeth are tightly occluded. Therefore, it is likely that some Class II malocclusions provide joint stability (a stable malocclusion) while others do not; but the same can be said for every type of static malocclusion category.
It is obvious that no simple relationship exists between orthopedic instability and intracapsular disorders. It is very important, however, that when orthopedic instability exists, it be identified as a potential etiologic factor. It should be noted that orthodontic therapy can be a viable treatment for orthopedic instability and may need to be considered when this instability has been determined to be a contributing factor to a TMD.
2.3.2.2 Osteoarthritis
When internal derangements of the TMJ occur, the structures affected by these changes often respond. The most common tissues affected are the retrodiscal tissues and the articular surfaces of the condyle and articular eminence. These changes can result in adaptation or destruction, depending upon many factors. Some of these factors are the acuteness of the changes as well as the intensity and duration of the loading. There are also important biologic and genetic factors that may regulate the patient’s ability to repair tissues.
If the articular surfaces of the condyle become affected, the subarticular bone receives additional loading and changes can occur. Similar changes also can occur in the absence of internal derangements. These changes represent a group of disorders that are considered joint arthritides. The most common type of TMJ arthritis is osteoarthritis (sometimes called degenerative joint disease). Osteoarthritis represents a destructive process by which the bony articular surfaces of the condyle and fossa become altered. It is generally considered to be the body’s response to increased loading of a joint [95]. As loading forces continue and the articular surface becomes softened (chondromalacia), the subarticular bone begins to resorb. Progressive degeneration eventually results in loss of the subchondral cortical layer, bone erosion, and subsequent radiographic evidence of osteoarthritis [96]. It is important to note that radiographic changes are only seen in later stages of osteoarthritis and may not reflect the clinical symptoms accurately.
Osteoarthritis is often painful, and jaw movement accentuates the symptoms. Crepitation (multiple grating joint sounds) is a common finding with this disorder. Osteoarthritis can occur any time the joint is overloaded, but is most commonly associated with disc displacements [97, 98] or perforation [99]. Once the disc is displaced and the retrodiscal tissues break down, the condyle begins to articulate directly with the fossa accelerating the destructive process. In time, the dense fibrous articular surfaces are destroyed and bony changes occur. Radiographically, the surfaces seem to be eroded and flattened. Any movement of these surfaces creates pain, so jaw function usually becomes very restricted. Although osteoarthritis is in the category of inflammatory disorders, it is not a true inflammatory condition. With appropriate treatment and reduction of joint loading, the arthritic condition can become adaptive. The adaptive stage has been referred to as osteoarthrosis [95, 100].
Other types of arthritides can certainly affect the temporomandibular joint. The most common after osteoarthritis is rheumatoid arthritis. This is thought to be an autoimmune disorder and therefore has its origin in systemic factors. The juvenile form of this problem can produce significant joint changes as well as serious occlusal problems. Other common causes of TMJ arthritis are traumatic arthritis, infectious arthritis, psoriatic arthritis, and hyperuricemia (gout). These conditions are not reviewed in this chapter. Other texts can be reviewed for a more thorough review of TMJ arthritides [2, p. 317–61].
2.4 Summary of the Continuum of TMJ Intracapsular Conditions
Disorders of the temporomandibular joints may follow a path of progressive events, i.e., a continuum, from the initial signs of dysfunction to osteoarthritis. They are summarized in Fig. 2.6.

Fig. 2.6
Various states of internal derangement of the TMJ. (a) normal joint, (b) partial displacement of the disc, (c) complete displacement of the disc, (d) impingement of retrodiscal tissues, (e) retrodiscitis and tissue breakdown, (f) osteoarthritis (From Okeson [2], p. 156)
Although this continuum is logical, the question must be asked whether these stages are always progressive for every patient. It is a question of great significance, because if all patients continue to progress in this manner, then there would be a professional obligation to resolve any joint symptoms as soon as they first appear. The sequence of breakdown as summarized in Fig. 2.6 is logical and has clinical support [101–103]. However, clinical longitudinal studies have clearly shown that some intracapsular TMD patients will present in one stage but may not necessarily progress to the next. At any given stage of disc derangement the patient may reach a level of adaptability and no further progression or breakdown will occur [104, 105]. This can be supported by histories of asymptomatic single and reciprocal clicks over many years [106]. Perhaps the key to determining who needs treatment lies in the obvious progression from one stage to the next. Also the presence of pain is important, since it implies continuous breakdown; in any case, the pain should be treated for its own sake. Therefore, it is this author’s opinion that treatment for such patients needs to be instituted when pain is associated with the condition. The treatment should be directed toward controlling pain and changing loading, thereby allowing a better opportunity for the tissues to repair and eventually adapt. Treatment of these conditions is beyond the objectives of this chapter and therefore other sources should be pursued.
Conclusion
Temporomandibular disorders are common conditions that may be encountered by orthodontists: during pre-treatment screening, during their treatment procedures, or during orthodontic retention. Every orthodontist needs to have a basic understanding of these musculoskeletal disorders so that he or she can respond to their patients’ needs. The purpose of this chapter was to present the pathophysiology, etiology, and clinical characteristics of the most common temporomandibular disorders. The goal was not to provide detailed therapeutic considerations for TMDs. However, it should be mentioned that most TMDs can be successfully managed by very conservative, reversible treatments. This should always be the initial approach before any irreversible treatments are considered.
Although some patients with TMDs may respond to orthodontic therapy, most will not because their occlusal conditions are not the cause of their symptomatology. Also, the positive responses seen may simply be due to placebo effects, spontaneous improvements, or the passage of time. Therefore, the orthodontist needs to understand which TMD patients will benefit before any orthodontic therapy is begun, and that treatment should not be initiated until acute symptoms of pain and dysfunction have been addressed. A review of the five etiologic factors presented in this chapter reveals that orthodontic therapy potentially affects only one of them, i.e., the occlusal condition. The manner by which orthodontic therapy affects this factor is by providing orthopedic stability, because proper orthodontic therapy can provide a stable occlusal position in the most stable joint position. Accomplishing this provides orthopedic stability in the masticatory structures, which minimizes risk factors associated with TMDs. Since orthodontic treatment will always disrupt the existing occlusal and TMJ relationships, establishing this orthopedic stability at the end of treatment should be the goal for every patient who receives orthodontic therapy.
When a patient presents to the orthodontist with a TMD, the etiology of the TMD needs to be determined before any treatment is begun. Assuming that the patient’s malocclusion is the major etiologic factor causing the TMD is a very naïve assumption. Nothing is more discouraging to the patient (and doctor) than to provide excellent orthodontic treatment for 2 years and then hear the patient report that the pain is still present. Although the orthodontic therapy may have been successful, the orthodontist has failed to successfully treat the patient. Therefore, before beginning orthodontic therapy on any symptomatic patient, the clinician needs to confirm that orthopedic instability is an etiology for that patient, because this is the only scenario in which orthodontics can be considered as an appropriate treatment for TMD.
Take Home Messages
- TMD signs and symptoms are common in the general population, but only a small percentage of those require treatment.
- Orthodontists need to be aware how their treatments can affect masticatory function.
- There are five recognized etiologic factors associated with TMD.
- Muscle pain is the most common painful TMD encountered in the orthodontic practice.
- Internal derangements are associated with TMJ conditions and joint sounds are the most common sign.
- Most TMD symptoms can be managed by conservative approaches.
- Treatment goals for all orthodontists should include developing or maintaining orthopedic stability in the masticatory system.
References
1.
de Leeuw R, Glasser G. Orofacial pain: guidelines for classification, assessment, and management. 5th ed. Chicago: Quintessence Publ. Co.; 2013.
2.
Okeson JP. Management of temporomandibular disorders and occlusion. 7th ed. St. Louis: Elsevier/Mosby Publishers; 2013.
3.
De Kanter RJAM, Kayser AF, Battistuzzi PGFCM, Truin GJ, Van T, Hol MA. Demand and need for treatment of craniomandibular dysfunction in the Dutch adult population. J Dent Res. 1992;71:1607–12.PubMed
4.
Schiffman EL, Fricton JR, Haley DP, Shapiro BL. The prevalence and treatment needs of subjects with temporomandibular disorders. J Am Dent Assoc. 1990;120(3):295–303.PubMed
5.
Pullinger AG, Seligman DA. The degree to which attrition characterizes differentiated patient groups of temporomandibular disorders. J Orofac Pain. 1993;7(2):196–208.PubMed
6.
Carlson CR, Okeson JP, Falace DA, Nitz AJ, Curran SL, Anderson DT. Comparison of psychologic and physiologic functioning between patients with masticatory muscle pain and matched controls. J Orofac Pain. 1993;7:15–22.PubMed
7.
Grassi C, Passatore M. Action of the sympathetic system on skeletal muscle. Ital J Neurol Sci. 1988;9(1):23–8.PubMed
8.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 13–40.
9.
Kobs G, Bernhardt O, Kocher T, Meyer G. Oral parafunctions and positive clinical examination findings. Stomatologija/issued by public institution “Odontologijos studija” [et al]. 2005;7(3):81–3.
10.
Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain Off J Am Pain Soc. 2011;12(11 Suppl):T92–101.
11.
Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol Disord Drug Targets. 2012;11(3):222–35.PubMedCentralPubMed
12.
Slade GD, Diatchenko L, Ohrbach R, Maixner W. Orthodontic treatment, genetic factors and risk of temporomandibular disorder. Semin Orthod. 2008;14(2):146–56.PubMedCentralPubMed
13.
McNamara Jr JA, Seligman DA, Okeson JP. Occlusion, orthodontic treatment, and temporomandibular disorders: a review. J Orofac Pain. 1995;9(1):73–90.PubMed
14.
McCreary CP, Clark GT, Merril RL, et al. Psychological distress and diagnostic subgroups of temporomandibular disorder patients. Pain. 1991;44:29–34.PubMed
15.
Keele KD. A physician looks at pain. In: Weisenberg M, editor. Pain; clinical and experimental perspectives. St Louis: The CV Mosby Co; 1975. p. 45–52.
16.
Svensson P, Graven-Nielsen T. Craniofacial muscle pain: review of mechanisms and clinical manifestations. J Orofac Pain. 2001;15(2):117–45.PubMed
17.
Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7(6):419–25.PubMed
18.
Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil. 2008;89(1):157–9.PubMed
19.
Mense S. Algesic agents exciting muscle nociceptors. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2009;196(1):89–100.PubMed
20.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 41–54.
21.
Lund JP, Widmer CG. Evaluation of the use of surface electromyography in the diagnosis, documentation, and treatment of dental patients. J Craniomandib Disord. 1989;3(3):125–37.PubMed
22.
Lund JP, Widmer CG, Feine JS. Validity of diagnostic and monitoring tests used for temporomandibular disorders [see comments]. J Dent Res. 1995;74(4):1133–43.PubMed
23.
Paesani DA, Tallents RH, Murphy WC, Hatala MP, Proskin HM. Evaluation of the reproducibility of rest activity of the anterior temporal and masseter muscles in asymptomatic and symptomatic temporomandibular subjects. J Orofac Pain. 1994;8:402–6.PubMed
24.
Curran SL, Carlson CR, Okeson JP. Emotional and physiologic responses to laboratory challenges: patients with temporomandibular disorders versus matched control subjects. J Orofac Pain. 1996;10(2):141–50.PubMed
25.
Mense S. Considerations concerning the neurobiological basis of muscle pain. Can J Physiol Pharmacol. 1991;69(5):610–6.PubMed
26.
Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54(3):241–89.PubMed
27.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 287–326.
28.
Watanabe M, Tabata T, Huh JI, Inai T, Tsuboi A, Sasaki K, et al. Possible involvement of histamine in muscular fatigue in temporomandibular disorders: animal and human studies. J Dent Res. 1999;78(3):769–75.PubMed
29.
Christensen LV, Mohamed SE, Harrison JD. Delayed onset of masseter muscle pain in experimental tooth clenching. J Prosthet Dent. 1982;48(5):579–84.PubMed
30.
Abraham WM. Factors in delayed muscle soreness. Med Sci Sports. 1977;9:11–20.PubMed
31.
Tegeder L, Zimmermann J, Meller ST, Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res Off J Europ Histamine Res Soc [et al]. 2002;51(8):393–402.
32.
Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev. 1991;19:99–125.PubMed
33.
Byrnes WC, Clarkson PM. Delayed onset muscle soreness and training. Clin Sports Med. 1986;5(3):605–14.PubMed
34.
Bobbert MF, Hollander AP, Huijing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18(1):75–81.PubMed
35.
Bakke M, Michler L. Temporalis and masseter muscle activity in patients with anterior open bite and craniomandibular disorders. Scand J Dent Res. 1991;99(3):219–28.PubMed
36.
Tzakis MG, Dahlstrom L, Haraldson T. Evaluation of masticatory funciton before and after treatment in patients with craniomandibular disorders. J Craniomandib Disord Facial Oral Pain. 1992;6:267–72.
37.
Sinn DP, de Assis EA, Throckmorton GS. Mandibular excursions and maximum bite forces in patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 1996;54(6):671–9.PubMed
38.
High AS, MacGregor AJ, Tomlinson GE. A gnathodynanometer as an objective means of pain assessment following wisdom tooth removal. Br J Maxillofac Surg. 1988;26:284.
39.
Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985;60(6):615–23.PubMed
40.
Simons DG, Travell J. Myofascial trigger points, a possible explanation [letter]. Pain. 1981;10(1):106–9.PubMed
41.
Mense S, Meyer H. Bradykinin-induced sensitization of high-threshold muscle receptors with slowly conducting afferent fibers. Pain. 1981;(Suppl 1):S204.
42.
Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75(1):1–17.PubMed
43.
McMillan AS, Blasberg B. Pain-pressure threshold in painful jaw muscles following trigger point injection. J Orofac Pain. 1994;8(4):384–90.PubMed
44.
Travell J. Introductory comments. In: Ragan C, editor. Connective tissues transactions of the fifth conference. New York: Josiah Macy, Jr; 1954. p. 12–22.
45.
Simons DG, Travell JG, Simons LS. Travell & Simons’ myofascial pain and dysfunction: a trigger point manual. 2nd ed. Baltimore: Williams & Wilkins; 1999. p. 67–78.
46.
Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18(13):1803–7.PubMed
47.
Fernandez-de-Las-Penas C, Galan-Del-Rio F, Alonso-Blanco C, Jimenez-Garcia R, Arendt-Nielsen L, Svensson P. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disorders. J Pain Off J Am Pain Soc. 2010;11(12):1295–304.
48.
Giunta JL, Kronman JH. Orofacial involvement secondary to trapezius muscle trauma. Oral Surg Oral Med Oral Pathol. 1985;60(4):368–9.PubMed
49.
Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. 2000;131(9):1307–15.PubMed
50.
Farrar WB, McCarty Jr WL. The TMJ dilemma. J Ala Dent Assoc. 1979;63(1):19–26.PubMed
51.
Roberts CA, Tallents RH, Espeland MA, Handelman SL, Katzberg RW. Mandibular range of motion versus arthrographic diagnosis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1985;60(3):244–51.PubMed
52.
Tallents RH, Hatala M, Katzberg RW, Westesson PL. Temporomandibular joint sounds in asymptomatic volunteers. J Prosthet Dent. 1993;69:298–304.PubMed
53.
Dibbets JMH, van der Weele LT. The prevalence of joint noises as related to age and gender. J Craniomandib Disord Facial Oral Pain. 1992;6:157–60.
54.
Katzberg RW, Westesson PL, Tallents RH, Drake CM. Anatomic disorders of the temporomandibular joint disc in asymptomatic subjects. J Oral Maxillofac Surg. 1996;54:147–53.PubMed
55.
Isberg A, Isacsson G, Johansson AS, Larson O. Hyperplastic soft-tissue formation in the temporomandibular joint associated with internal derangement. A radiographic and histologic study. Oral Surg Oral Med Oral Pathol. 1986;61(1):32–8.PubMed
56.
Holumlund AB, Gynther GW, Reinholt FP. Disk derangement and inflammatory changes in the posterior disk attachment of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1992;73:9.
57.
Taskaya-Yylmaz N, Ogutcen-Toller M. Clinical correlation of MRI findings of internal derangements of the temporomandibular joints. Br J Oral Maxillofac Surg. 2002;40(4):317–21.PubMed
58.
Harkins SJ, Marteney JL. Extrinsic trauma: a significant precipitating factor in temporomandibular dysfunction. J Prosthet Dent. 1985;54(2):271–2.PubMed
59.
Moloney F, Howard JA. Internal derangements of the temporomandibular joint. III. Anterior repositioning splint therapy. Aust Dent J. 1986;31(1):30–9.PubMed
60.
Weinberg S, Lapointe H. Cervical extension-flexion injury (whiplash) and internal derangement of the temporomandibular joint. J Oral Maxillofac Surg. 1987;45(8):653–6.PubMed
61.
Pullinger AG, Seligman DA. Trauma history in diagnostic groups of temporomandibular disorders. Oral Surg Oral Med Oral Pathol. 1991;71(5):529–34.PubMed
62.
Westling L, Carlsson GE, Helkimo M. Background factors in craniomandibular disorders with special reference to general joint hypermobility, parafunction, and trauma. J Craniomandib Disord. 1990;4(2):89–98.PubMed
63.
Pullinger AG, Seligman DA. Association of TMJ subgroups with general trauma and MVA. J Dent Res. 1988;67:403.
64.
Pullinger AG, Monteriro AA. History factors associated with symptoms of temporomandibular disorders. J Oral Rehabil. 1988;15:117.PubMed
65.
Skolnick J, Iranpour B, Westesson PL, Adair S. Prepubertal trauma and mandibular asymmetry in orthognathic surgery and orthodontic paients. Am J Orthod Dentofacial Orthop. 1994;105:73–7.PubMed
66.
Braun BL, DiGiovanna A, Schiffman E, et al. A cross-sectional study of temporomandibular joint dysfunction in post-cervical trauma patients. J Craniomandib Disord Facial Oral Pain. 1992;6:24–31.
67.
Burgess J. Symptom characteristics in TMD patients reporting blunt trauma and/or whiplash injury. J Craniomandib Disord. 1991;5(4):251–7.PubMed
68.
De Boever JA, Keersmaekers K. Trauma in patients with temporomandibular disorders: frequency and treatment outcome. J Oral Rehabil. 1996;23(2):91–6.PubMed
69.
Yun PY, Kim YK. The role of facial trauma as a possible etiologic factor in temporomandibular joint disorder. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2005;63(11):1576–83.
70.
Arakeri G, Kusanale A, Zaki GA, Brennan PA. Pathogenesis of post-traumatic ankylosis of the temporomandibular joint: a critical review. Br J Oral Maxillofac Surg. 2012;50(1):8–12.PubMed
71.
Okeson JP. Bell’s oral and facial pain. 7th ed. Chicago: Quintessence Publishers; 2014. p. 327–69.
72.
Israel HA, Diamond B, Saed Nejad F, Ratcliffe A. The relationship between parafunctional masticatory activity and arthroscopically diagnosed temporomandibular joint pathology. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 1999;57(9):1034–9.
73.
Nitzan DW. Intraarticular pressure in the functioning human temporomandibular joint and its alteration by uniform elevation of the occlusal plane. J Oral Maxillofac Surg. 1994;52(7):671–9.PubMed
74.
Milam SB, Schmitz JP. Molecular biology of temporomandibular joint disorders: proposed mechanisms of disease. J Oral Maxillofac Surg. 1995;12:1448–54.
75.
Monje F, Delgado E, Navarro MJ, Miralles C, Alonso Jr dH. Changes in temporomandibular joint after mandibular subcondylar osteotomy: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:1221–34.PubMed
76.
Shaw RM, Molyneux GS. The effects of induced dental malocclusion on the fibrocartilage disc of the adult rabbit temporomandibular joint. Arch Oral Biol. 1993;38:415–22.PubMed
77.
Isberg A, Isacsson G. Tissue reactions associated with internal derangement of the temporomandibular joint. A radiographic, cryomorphologic, and histologic study. Acta Odontol Scand. 1986;44(3):160–4.PubMed
78.
Wright Jr WJ. Temporomandibular disorders: occurrence of specific diagnoses and response to conservative management. Clin Observ Craniol. 1986;4(2):150–5.
79.
Seligman DA, Pullinger AG. Association of occlusal variables among refined TM patient diagnostic groups. J Craniomandib Disord. 1989;3(4):227–36.PubMed
80.
Solberg WK, Bibb CA, Nordstrom BB, Hansson TL. Malocclusion associated with temporomandibular joint changes in young adults at autopsy. Am J Orthod. 1986;89(4):326–30.PubMed
81.
Tsolka P, Walter JD, Wilson RF, Preiskel HW. Occlusal variables, bruxism and temporomandibulae disorder: a clinical and kinesiographic assessment. J Oral Rehabil. 1995;22:849–956.PubMed
82.
Celic R, Jerolimov V. Association of horizontal and vertical overlap with prevalence of temporomandibular disorders. J Oral Rehabil. 2002;29(6):588–93.PubMed
83.
Williamson EH, Simmons MD. Mandibular asymmetry and its relation to pain dysfunction. Am J Orthod. 1979;76(6):612–7.PubMed
84.
DeBoever JA, Adriaens PA. Occlusal relationship in patients with pain-dysfunction symptoms in the temporomandibular joint. J Oral Rehabil. 1983;10:1–7.
85.
Brandt D. Temporomandibular disorders and their association with morphologic malocclusion in children. In: Carlson DS, McNamara JA, Ribbens KA, editors. Developmental aspects of temporomandibular joint disorders. Ann Arbor: University of Michigan Press; 1985. p. 279.
86.
Nilner M. Functional disturbances and diseases of the stomatognathic system. A cross-sectional study. J Pedod. 1986;10(3):211–38.PubMed
87.
Stringert HG, Worms FW. Variations in skeletal and dental patterns in patients with structural and functional alterations of the temporomandibular joint: a preliminary report. Am J Orthod. 1986;89(4):285–97.PubMed
88.
Gunn SM, Woolfolk MW, Faja BW. Malocclusion and TMJ symptoms in migrant children. J Craniomandib Disord. 1988;2(4):196–200.PubMed
89.
Dworkin SF, Huggins KH, LeResche L, Von KM, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120(3):273–81.PubMed
90.
Ronquillo HI, et al. Comparison of internal deranagements with condyle-fossa relationship, horizontal and vertical overlap, and angle class. J Craniomandib Disord Facial Oral Pain. 1988;2:137.
91.
Pullinger AG, Seligman DA, Solberg WK. Temporomandibular disorders. Part II: occlusal factors associated with temporomandibular joint tenderness and dysfunction. J Prosthet Dent. 1988;59(3):363–7.PubMed
92.
Pullinger AG, Seligman DA. Overbite and overjet characteristics of refined diagnositic groups of temporomandibular disorders patients. Am J Orthod Dentofacial Orthop. 1991;100:401.PubMed
93.
Hirsch C, John MT, Drangsholt MT, Mancl LA. Relationship between overbite/overjet and clicking or crepitus of the temporomandibular joint. J Orofac Pain. 2005;19(3):218–25.PubMed
94.
John MT, Hirsch C, Drangsholt MT, Mancl LA, Setz JM. Overbite and overjet are not related to self-report of temporomandibular disorder symptoms. J Dent Res. 2002;81(3):164–9.PubMed
95.
Stegenga B, de Bont L, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg. 1989;47(3):249–56.PubMed
96.
Stegenga B, de Bont LG, Boering G, van Willigen JD. Tissue responses to degenerative changes in the temporomandibular joint: a review. J Oral Maxillofac Surg. 1991;49(10):1079–88.PubMed
97.
DeBont LGM, Boering G, Liem RSB, Eulderink F, Westesson PL, et al. Osteoarthritis and internal derangement of the temporomandibular joint: a light microscopic study. J Oral Maxillofac Surg. 1986;44:634–43.
98.
Mills DK, Daniel JC, Herzog S, Scapino RP. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillofac Surg. 1994;52(12):1279–92.PubMed
99.
Helmy E, Bays R, Sharawy M. Osteoarthrosis of the temporomandibular joint following experimental disc perforation in Macaca fascicularis. J Oral Maxillofac Surg. 1988;46(11):979–90.PubMed
100.
Boering G. Temporomandibular joint arthrosis: a clinical and radiographic investigation [thesis]. Groningen: University of Groningen; 1966.
101.
Farrar WB, McCarty WL. A clinical outline of temporomandibular joint diagnosis and treatment. 7th ed. Montgomery: Normandie Publications; 1983. p. 191.
102.
McCarty WL, Farrar WB. Surgery for internal derangements of the temporomandibular joint. J Prosthet Dent. 1979;42(2):191–6.PubMed
103.
Wilkes CH. Arthrography of the temporomandibular joint in patients with the TMJ pain-dysfunction syndrome. Minn Med. 1978;61(11):645–52.PubMed
104.
Akerman S, Kopp S, Rohlin M. Histological changes in temporomandibular joints from elderly individuals. An autopsy study. Acta Odontol Scand. 1986;44(4):231–9.PubMed
105.
Kircos LT, Ortendahl DA, Mark AS, Arakawa M. Magnetic resonance imaging of the TMJ disc in asymptomatic volunteers. J Oral Maxillofac Surg. 1987;45(10):852–4.PubMed
106.
Magnusson T, Egermark I, Carlsson GE. A longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of age. J Orofac Pain. 2000;14(4):310–9.PubMed